Online Database of Chemicals from Around the World
| SLN Pharmachem | India | Inquire | ||
|---|---|---|---|---|
 |
+91 (22) 2870-1926 6524-0421 2854-9125 | |||
 |
ho@slnpharmachem.com office@slnpharmachem.co.in | |||
| Chemical manufacturer since 1997 | ||||
| chemBlink standard supplier since 2008 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Shanghai Amino-Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 5030-6558 | |||
 |
stepheni@amino-chem.cn market@amino-chem.cn | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2009 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| Hangzhou Leap Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8771-1850 | |||
 |
market19@leapchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2015 | ||||
| Weifang Fine Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (536) 517-0190 | |||
 |
1297429207@qq.com | |||
| Chemical manufacturer since 2014 | ||||
| chemBlink standard supplier since 2016 | ||||
| Hangzhou Molcore Biopharmatech Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8102-5280 | |||
 |
sales@molcore.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2010 | ||||
| chemBlink standard supplier since 2017 | ||||
| Changzhou Hongyu Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (519) 8999-6110 | |||
 |
545701991@qq.com | |||
| Chemical distributor since 2011 | ||||
| chemBlink standard supplier since 2018 | ||||
| Classification | Pharmaceutical intermediate >> Heterocyclic compound intermediate >> Piperazine |
|---|---|
| Name | 1-Methylpiperazine |
| Synonyms | 1-Methyl piperazine; Methylpiperazine; N-Methyl piperazine |
| Molecular Structure | 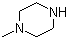 |
| Molecular Formula | C5H12N2 |
| Molecular Weight | 100.16 |
| CAS Registry Number | 109-01-3 |
| EC Number | 203-639-5 |
| SMILES | CN1CCNCC1 |
| Density | 0.9±0.1 g/cm3 Calc.*, 0.903 g/mL (Expl.) |
|---|---|
| Melting point | -6 ºC (Expl.) |
| Boiling point | 138.0±8.0 ºC 760 mmHg (Calc.)*, 138 ºC (Expl.) |
| Flash point | 42.2 ºC (Calc.)*, 34 ºC (Expl.) |
| Index of refraction | 1.442 (Calc.)*, 1.466 (Expl.) |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |



 GHS02;GHS05;GHS06;GHS07 DangerGHS02 Details
GHS02;GHS05;GHS06;GHS07 DangerGHS02 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H226-H312-H314-H317-H318-H330-H331-H332 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P210-P233-P240-P241-P242-P243-P260-P261-P264-P264+P265-P271-P272-P280-P284-P301+P330+P331-P302+P352-P302+P361+P354-P303+P361+P353-P304+P340-P305+P354+P338-P316-P317-P320-P321-P333+P317-P362+P364-P363-P370+P378-P403+P233-P403+P235-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Transport Information | UN 2734 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
1‑Methylpiperazine is a heterocyclic organic compound belonging to the class of piperazine derivatives. It consists of a six-membered saturated ring containing two nitrogen atoms at opposite positions (1,4-diazacyclohexane), with a methyl group attached to one of the nitrogen atoms. The molecular formula of 1‑methylpiperazine is C5H12N2, and its structure imparts basicity and nucleophilicity due to the presence of the secondary and tertiary amine groups. This compound is a colorless liquid under standard conditions and exhibits a characteristic amine odor. The discovery and initial study of piperazine derivatives date back to the late 19th and early 20th centuries when chemists were exploring heterocyclic nitrogen-containing compounds for pharmaceutical and chemical applications. The methylation of one nitrogen atom in piperazine to form 1‑methylpiperazine was developed to modify the chemical reactivity and physical properties of the parent piperazine molecule. The N-methyl substitution increases the lipophilicity and slightly reduces the basicity of the substituted nitrogen compared to the unsubstituted secondary amine, making the compound more suitable for applications in organic synthesis and pharmaceutical research. 1‑Methylpiperazine is widely used as a building block in the synthesis of numerous pharmaceuticals, agrochemicals, and functionalized materials. Its structure allows it to serve as a nucleophilic site for reactions with electrophiles such as alkyl halides, acyl halides, and sulfonyl chlorides. In medicinal chemistry, 1‑methylpiperazine is frequently incorporated into drug molecules to provide solubility, enhance receptor binding, or act as a linker between pharmacophores. It has been used in the synthesis of central nervous system agents, antimalarial drugs, and other bioactive compounds where the piperazine moiety contributes to binding affinity and pharmacokinetic properties. In addition to pharmaceutical applications, 1‑methylpiperazine is employed in the preparation of chelating agents, polymer modifiers, and specialty solvents. The secondary and tertiary amine groups can coordinate with metal ions, which is useful in complexation chemistry or in the design of polymer networks. The compound’s liquid state at room temperature, combined with its relatively low viscosity, allows it to be used as a solvent or co-solvent in chemical reactions requiring a basic medium. The chemical reactivity of 1‑methylpiperazine is influenced by the presence of two nitrogen atoms with different substitution patterns. The methylated nitrogen is a tertiary amine, which cannot be protonated to form a primary ammonium salt but still provides steric hindrance and electron donation in reactions. The other nitrogen is a secondary amine, which can participate in protonation, hydrogen bonding, or nucleophilic substitution reactions. This dual functionality makes 1‑methylpiperazine versatile as both a nucleophile and a base in synthetic chemistry. Physically, 1‑methylpiperazine is miscible with water and many polar organic solvents, including alcohols, acetone, and dimethyl sulfoxide. Its boiling point is moderate, allowing it to be distilled under standard laboratory conditions. The compound is chemically stable under normal conditions but can react with strong oxidizing agents or acylating reagents, and it is hygroscopic, requiring proper storage in airtight containers to maintain purity. In industrial and research contexts, 1‑methylpiperazine is valued for its versatility, ease of handling, and reactivity. It can be used as a precursor to N-substituted piperazines, heterocyclic ligands, and polymer additives. The compound’s basicity and nucleophilicity make it a common reagent in the preparation of amides, ureas, and other functional groups, providing flexibility in synthetic strategy. Its incorporation into biologically active molecules demonstrates the importance of simple nitrogen heterocycles as scaffolds in chemical research. Overall, 1‑methylpiperazine is a fundamental heterocyclic amine with wide applicability in synthetic chemistry, pharmaceuticals, and materials science. Its unique combination of secondary and tertiary amine groups, combined with water solubility and moderate lipophilicity, enables diverse chemical transformations and functional applications, making it a valuable building block in modern chemistry. References 2025. Recent advances in pyrazolo[3,4-b]pyridine chemistry: synthesis techniques and biological activity. Molecular Diversity. DOI: 10.1007/s11030-025-11231-5 2025. Design, synthesis and antitumor activity of thiadiazole derivatives as novel ALK kinase inhibitors. Molecular Diversity. DOI: 10.1007/s11030-025-11259-7 |
| Market Analysis Reports |
| List of Reports Available for 1-Methylpiperazine |