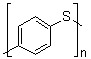
Poly(thio-1,4-phenylene) is a polymer composed of repeating 1,4-phenylene units linked through sulfur atoms. Its molecular formula can be represented as –[C6H4S]n–, where n indicates the number of repeating units. The polymer exhibits a rigid, conjugated backbone due to the aromatic rings and the alternating sulfur linkages, which impart both thermal stability and electronic delocalization along the chain. It typically appears as a dark-colored solid and is insoluble in water but can be processed in certain polar organic solvents depending on the molecular weight and the degree of polymerization.
The discovery of poly(thio-1,4-phenylene) stems from research into conductive and high-performance polymers in the 20th century. Its synthesis was motivated by the need for materials combining aromatic stability with the unique electronic properties of sulfur-containing linkages. The polymer’s conjugated structure enables delocalization of electrons, making it of interest in studies of conductive polymers, photoconductive materials, and electronic devices. Its chemical structure allows for strong π-π stacking interactions between polymer chains, which contributes to its mechanical strength and stability under heat or oxidative conditions.
Synthesis of poly(thio-1,4-phenylene) is commonly achieved through oxidative polymerization of 1,4-dithiols or via coupling reactions between 1,4-dihalobenzenes and sulfide sources. In the oxidative polymerization approach, 1,4-benzenedithiol is treated with an oxidizing agent under controlled temperature and solvent conditions, facilitating the formation of sulfur linkages between phenylene units. Alternative methods involve nucleophilic aromatic substitution reactions, where halogenated aromatic compounds react with sulfide anions to produce the polymeric chain. Reaction parameters such as solvent, temperature, and catalyst selection are carefully optimized to control molecular weight and polymer uniformity.
Chemically, poly(thio-1,4-phenylene) is resistant to hydrolysis and many oxidizing or reducing conditions, which makes it stable under a range of processing and application environments. The sulfur atoms in the backbone provide sites for further chemical modification, such as oxidation to sulfoxides or sulfones, which can tune the polymer’s solubility, thermal behavior, and electronic properties. The polymer’s aromatic and conjugated structure allows for interaction with light and electric fields, making it a candidate for optoelectronic applications.
In terms of applications, poly(thio-1,4-phenylene) is explored in areas including conductive and semiconductive materials, sensors, and high-performance composites. Its electronic properties can be harnessed in devices requiring electron transport, photoconduction, or charge storage. Additionally, the thermal and chemical stability of the polymer allows it to be used in coatings, membranes, and other materials where mechanical strength and resistance to degradation are essential. Modifications of the polymer backbone or incorporation into composite materials can further extend its utility in specialized applications.
Physical properties of poly(thio-1,4-phenylene) include a high melting or softening point, insolubility in water, and variable solubility in organic solvents depending on molecular weight. Its rigidity and aromatic content contribute to mechanical strength and structural integrity, while its sulfur linkages influence electronic conductivity. Processing typically involves solution casting, melt processing, or incorporation into composite matrices for desired performance characteristics.
Overall, poly(thio-1,4-phenylene) is a conjugated, sulfur-containing aromatic polymer notable for its chemical stability, electronic delocalization, and mechanical strength. Its synthesis through oxidative polymerization or coupling reactions enables the production of high-performance polymeric materials, while the sulfur linkages provide sites for functional modification. The combination of thermal stability, electronic properties, and structural rigidity makes it an important material for research and development in conductive polymers, optoelectronics, and high-performance polymer applications.
|