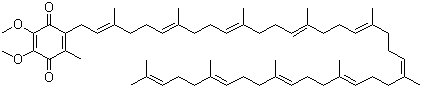
Ubidecarenone, also known as coenzyme Q10 or CoQ10, is a lipid-soluble benzoquinone derivative with a long isoprenoid side chain, which allows it to localize within biological membranes. Its molecular formula is C59H90O4. Ubidecarenone naturally occurs in the mitochondria of eukaryotic cells and plays a critical role in the electron transport chain, where it facilitates the transfer of electrons between complexes I and II to complex III. This function is essential for the generation of adenosine triphosphate (ATP), the primary energy currency of the cell.
The discovery of ubidecarenone stems from research on cellular respiration and energy metabolism. It was first identified as a coenzyme required for the activity of mitochondrial enzymes involved in oxidative phosphorylation. Its structure consists of a redox-active quinone ring and a hydrophobic isoprenoid tail, which anchors the molecule in the inner mitochondrial membrane, allowing efficient electron transfer. The quinone ring can undergo reversible reduction and oxidation, cycling between the oxidized form (ubiquinone) and the reduced form (ubiquinol), which is critical for its function as an electron carrier.
Ubidecarenone can be synthesized chemically or obtained through extraction from natural sources such as animal tissues and certain microorganisms. Chemical synthesis involves the condensation of a hydroquinone derivative with an isoprenoid chain using controlled conditions to maintain the integrity of the quinone and avoid side reactions. Biosynthetic pathways in organisms produce ubidecarenone through a sequence of enzymatic reactions starting from mevalonate, leading to the formation of the isoprenoid tail, which is then linked to the quinone head group.
Chemically, ubidecarenone is stable under dry, ambient conditions but sensitive to light, heat, and strong oxidizing agents. Its redox activity enables it to function as both an electron carrier and an antioxidant, neutralizing reactive oxygen species and protecting cellular components from oxidative damage. The compound’s lipophilicity allows it to diffuse within lipid membranes, enhancing its ability to interact with multiple enzymatic complexes and participate in membrane-bound electron transport processes.
In addition to its physiological role, ubidecarenone has numerous applications in medicine and nutraceuticals. It is used as a dietary supplement to support mitochondrial function, particularly in individuals with mitochondrial disorders, cardiovascular disease, or age-related decline in CoQ10 levels. Its antioxidant properties contribute to the protection of lipids, proteins, and DNA from oxidative stress. Pharmaceutical formulations may include ubidecarenone in oil-based capsules or as part of liposomal preparations to improve bioavailability, given its poor water solubility.
From a physical perspective, ubidecarenone is a yellow to orange crystalline solid or viscous oil depending on formulation. It is soluble in lipids, ethanol, and other organic solvents but poorly soluble in water, necessitating careful formulation strategies for oral or topical delivery. Safety considerations include avoiding excessive exposure to oxidizing conditions and protecting the compound from prolonged light, which can degrade its quinone functionality.
Overall, ubidecarenone is a biologically and chemically important benzoquinone derivative that functions as an essential electron carrier in the mitochondrial electron transport chain and as an antioxidant. Its structural combination of a redox-active quinone ring and a hydrophobic isoprenoid tail underlies its physiological and therapeutic significance. Ubidecarenone continues to be a critical molecule in studies of bioenergetics, oxidative stress, and clinical interventions aimed at supporting cellular energy metabolism and protecting against oxidative damage.
References
2025. Modulating lipid metabolism for enhancement of coenzyme Q10 production in Rhodobacter sphaeroides. Bioresource Technology.
DOI: 10.1016/j.biortech.2025.132794
|


















 GHS07 Warning Details
GHS07 Warning Details