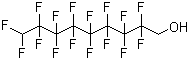
1H,1H,9H-Hexadecafluoro-1-nonanol is a perfluorinated alcohol consisting of a nine-carbon linear chain in which 16 hydrogen atoms are replaced by fluorine, except for a terminal hydroxyl group. Its molecular formula is C9H3F16O. The compound appears as a colorless to pale liquid with low surface tension, high chemical and thermal stability, and exceptional resistance to oxidation and hydrolysis. Its highly fluorinated chain renders it both hydrophobic and lipophobic, while the terminal hydroxyl group provides limited polarity and the ability to participate in hydrogen bonding.
The discovery and use of perfluorinated alcohols like 1H,1H,9H-hexadecafluoro-1-nonanol are rooted in the development of fluorinated surfactants and specialty chemicals for industrial and materials applications. The strong carbon-fluorine bonds in the perfluorinated chain confer chemical inertness, thermal stability, and resistance to degradation, which make the compound useful in coatings, surface treatments, and as a precursor for fluorinated polymers. The terminal hydroxyl group allows chemical modification to introduce reactive functionalities for further synthetic applications.
Synthesis of 1H,1H,9H-hexadecafluoro-1-nonanol generally involves the partial fluorination of linear alcohol precursors using elemental fluorine, cobalt or nickel-catalyzed electrochemical fluorination, or via telomerization reactions starting from tetrafluoroethylene. The selective introduction of fluorine while retaining the terminal hydroxyl group requires precise reaction control and often involves multi-step strategies. Purification is usually performed by distillation under reduced pressure to remove unreacted precursors and side products.
Chemically, the compound is characterized by the strong electron-withdrawing effect of the perfluoroalkyl chain, which reduces nucleophilicity and increases the acidity of the hydroxyl proton relative to non-fluorinated alcohols. The hydroxyl group allows formation of esters, ethers, or other derivatives, enabling the incorporation of the fluorinated moiety into polymers, surfactants, or specialty coatings. The fluorinated chain provides low surface energy, chemical resistance, and thermal stability, which are highly desirable in industrial applications.
In practical applications, 1H,1H,9H-hexadecafluoro-1-nonanol is used as a fluorosurfactant, wetting agent, or intermediate in the synthesis of fluorinated esters, polymers, and surface-active materials. Its ability to lower surface tension and resist chemical attack makes it suitable for protective coatings, stain-resistant textiles, and specialty lubricants. It is also employed in the production of fluorinated monomers and oligomers, which are precursors for highly stable materials used in electronics, aerospace, and chemical-resistant surfaces.
Physically, the compound is stable under ambient conditions and has a high boiling point due to strong intermolecular interactions involving the hydroxyl group and the dense fluorine atoms. It is generally handled in closed systems or under inert atmospheres to prevent contamination and preserve its functional properties. Standard laboratory precautions should be observed, including gloves and eye protection, as perfluorinated compounds can be bioaccumulative and persistent in the environment.
Overall, 1H,1H,9H-hexadecafluoro-1-nonanol is a highly fluorinated alcohol with unique chemical and physical properties. Its combination of a reactive hydroxyl group and a chemically inert, hydrophobic perfluoroalkyl chain makes it valuable as a building block for surfactants, polymers, and specialty materials. The compound exemplifies the utility of partially fluorinated alcohols in applications requiring chemical resistance, low surface energy, and thermal stability.
References
2013. Biocidal activity of the esterification products of polyfluoroalkyl alcohols and pentafluorophenol with resin acids. Russian Journal of General Chemistry, 83(13).
DOI: 10.1134/s1070363213130203
|









 GHS07;GHS08 Danger Details
GHS07;GHS08 Danger Details