Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Natural product >> Flavonoids |
|---|---|
| Name | Oroxylin A |
| Synonyms | Oroxylin; 5,7-Dihydroxy-6-methoxyflavone; 6-Methoxybaicalein; Baicalein 6-methyl ether |
| Molecular Structure | 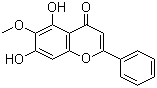 |
| Molecular Formula | C16H12O5 |
| Molecular Weight | 284.26 |
| CAS Registry Number | 480-11-5 |
| EC Number | 663-302-7 |
| SMILES | COC1=C(C2=C(C=C1O)OC(=CC2=O)C3=CC=CC=C3)O |
| Density | 1.4±0.1 g/cm3 Calc.* |
|---|---|
| Melting point | 195-197 ºC (Expl.) |
| Boiling point | 540.9±50.0 ºC 760 mmHg (Calc.)* |
| Flash point | 207.4±23.6 ºC (Calc.)* |
| Solubility | DMSO, 10 mM (Expl.) |
| Index of refraction | 1.669 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302 Details | ||||||||||||
| Precautionary Statements | P264-P270-P301+P317-P330-P501 Details | ||||||||||||
| Hazard Classification | |||||||||||||
| |||||||||||||
| SDS | Available | ||||||||||||
|
Oroxylin A is a naturally occurring flavonoid predominantly found in the roots of Scutellaria baicalensis Georgi, a medicinal plant widely used in traditional East Asian medicine. Chemically, it is a methoxylated flavone, structurally related to other bioactive flavonoids such as baicalein and wogonin. Oroxylin A has attracted sustained scientific interest due to its well-documented pharmacological activities and its role as a representative compound linking traditional herbal use with modern biochemical and pharmacological research. The discovery of oroxylin A is closely associated with phytochemical investigations of Scutellaria species conducted during the twentieth century. As analytical techniques such as chromatography and spectroscopy became more advanced, researchers were able to isolate and characterize individual flavonoids responsible for the biological effects of these medicinal plants. Oroxylin A was identified as one of the major aglycone constituents produced through hydrolysis of its glycosidic precursors present in the plant material. Its isolation helped clarify the chemical basis underlying the therapeutic reputation of Scutellaria-based preparations. Structurally, oroxylin A is a flavone bearing hydroxyl groups and a methoxy substituent on the aromatic rings. This arrangement contributes to its physicochemical properties, including moderate lipophilicity and the ability to participate in hydrogen bonding and redox reactions. The flavone backbone allows interaction with a variety of biological targets, including enzymes, receptors, and signaling proteins. These structural features are central to its observed bioactivities and have made oroxylin A a useful scaffold for structure–activity relationship studies. The applications of oroxylin A have been extensively explored in pharmacological and biomedical research. It has been shown to exhibit anti-inflammatory activity by modulating key signaling pathways involved in inflammatory responses, including the regulation of cytokine production and inhibition of pro-inflammatory transcription factors. These properties are consistent with the traditional use of Scutellaria extracts in treating inflammatory and febrile conditions. Oroxylin A has also demonstrated antioxidant activity, contributing to cellular protection against oxidative stress. In oncology-related research, oroxylin A has been studied for its effects on cancer cell proliferation, apoptosis, and metastasis. Experimental studies have shown that it can influence cell cycle regulation and induce programmed cell death in various cancer cell lines. Additionally, it has been reported to affect tumor-associated signaling pathways, making it a compound of interest in the search for plant-derived anticancer agents. These findings have positioned oroxylin A as a promising lead compound for further preclinical investigation. Neurological applications represent another important area of research for oroxylin A. Studies have shown that it can cross the blood–brain barrier and exert neuroprotective effects. It has been investigated for its potential to improve cognitive function, modulate neurotransmitter systems, and reduce neuroinflammation. These properties have prompted interest in its possible relevance to neurodegenerative diseases and cognitive disorders. Beyond therapeutic research, oroxylin A is also used as a reference compound in analytical chemistry and natural product research. It serves as a standard for quality control of herbal medicines containing Scutellaria extracts and is employed in studies examining the metabolism, bioavailability, and transformation of flavonoids in biological systems. Overall, oroxylin A represents a well-characterized natural flavonoid whose discovery stemmed from traditional medicinal plants and whose applications span inflammation, cancer biology, neuroscience, and analytical science. Its study illustrates how natural products can bridge ethnopharmacology and modern biomedical research, providing chemically defined molecules with diverse and scientifically validated biological activities. References 2025. Astragalin promotes HSCs ferroptosis through NCOA4 mediated ferritinophagy to alleviate liver fibrosis in zebrafish and mice. Communications Biology. DOI: 10.1038/s42003-025-08421-0 2025. Oroxylin A alleviates pyroptosis and apoptosis in human corneal epithelial cells under hyperosmotic stress by activating the SIRT3-SOD2/HIF-1a pathway. Experimental Eye Research. DOI: 10.1016/j.exer.2025.110345 2025. Oroxylin A inhibits UVB-induced non-melanoma skin cancer by regulating XPA degradation. Chinese Journal of Natural Medicines. DOI: 10.1016/s1875-5364(25)60893-4 |
| Market Analysis Reports |
| List of Reports Available for Oroxylin A |