Online Database of Chemicals from Around the World
| Trademax Pharmaceuticals & Chemicals Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 5238-7770 | |||
 |
trademax@trademaxchem.com | |||
| Chemical distributor since 2001 | ||||
| chemBlink standard supplier since 2006 | ||||
| Capot Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8558-6718 +86 13336195806 | |||
 |
capotchem@gmail.com sales@capotchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2006 | ||||
| SinoRefine Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 5422-4052 | |||
 |
info@sinorefine.com | |||
| Chemical distributor | ||||
| chemBlink standard supplier since 2007 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Extrasynthese Chemical S.A.S. | France | Inquire | ||
|---|---|---|---|---|
 |
+33 (47) 898-2034 | |||
 |
info@extrasynthese.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2009 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Intatrade Chemicals GmbH | Germany | Inquire | ||
|---|---|---|---|---|
 |
+49 (3493) 605-465 | |||
 |
sales@intatrade.de | |||
| Chemical distributor | ||||
| chemBlink standard supplier since 2011 | ||||
| Classification | Biochemical >> Plant extracts |
|---|---|
| Name | Hinokitiol |
| Synonyms | 2-Hydroxy-4-isopropyl-cyclohepta-2,4,6-trien-1-one; 2-Hydroxy-4-(1-methylethyl)-2,4,6-cycloheptatrien-1-one; beta-Thujaplicin |
| Molecular Structure | 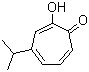 |
| Molecular Formula | C10H12O2 |
| Molecular Weight | 164.20 |
| CAS Registry Number | 499-44-5 |
| EC Number | 207-880-7 |
| SMILES | CC(C)C1=CC(=O)C(=CC=C1)O |
| Density | 1.1±0.1 g/cm3 Calc.* |
|---|---|
| Melting point | 50 - 52 ºC (Expl.) |
| Boiling point | 303.4±35.0 ºC 760 mmHg (Calc.)*, 306.2 ºC (Expl.) |
| Flash point | 128.1±18.5 ºC (Calc.)* |
| Index of refraction | 1.554 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302 Details | ||||||||||||||||
| Precautionary Statements | P264-P270-P301+P317-P330-P501 Details | ||||||||||||||||
| Hazard Classification | |||||||||||||||||
| |||||||||||||||||
| SDS | Available | ||||||||||||||||
|
Hinokitiol is a naturally occurring monoterpenoid characterized by a seven-membered aromatic ring bearing a hydroxyl group and an isopropyl substituent. It is a member of the tropolone family of compounds and is best known as a constituent of the essential oils of certain coniferous trees. The compound has attracted sustained scientific interest because of its distinctive chemical structure and its broad range of biological and practical applications. The discovery of hinokitiol is closely linked to early twentieth-century investigations of natural products derived from wood. During studies of the chemical components responsible for the durability and fragrance of Japanese cypress species, researchers isolated a crystalline compound from the heartwood. This substance was later identified as hinokitiol and shown to be a major contributor to the resistance of the wood against microbial decay. Its identification represented an important advance in understanding how specific organic compounds confer natural preservative properties to plant materials. Structurally, hinokitiol differs from more common phenolic compounds by possessing a non-benzenoid aromatic ring. The tropolone framework, with its conjugated system and intramolecular hydrogen bonding, gives hinokitiol unusual electronic properties. Early chemical studies focused on elucidating this structure and on understanding how its arrangement influenced acidity, metal-chelating ability, and reactivity. These investigations established hinokitiol as a representative example of a stable, naturally occurring non-benzenoid aromatic compound, expanding traditional concepts of aromaticity in organic chemistry. The applications of hinokitiol were initially rooted in its antimicrobial properties. Laboratory studies demonstrated that the compound could inhibit the growth of bacteria and fungi, findings that aligned with its natural role in protecting tree heartwood. These properties led to its use in products designed to prevent microbial contamination or spoilage. Hinokitiol has been incorporated into wood preservatives, coatings, and other materials where resistance to biological degradation is desirable. Beyond its use as a preservative, hinokitiol found applications in personal care and cosmetic products. Its antimicrobial activity, combined with a relatively mild sensory profile, supported its inclusion in formulations such as oral hygiene products, skin care preparations, and hair care items. In these contexts, hinokitiol has been valued for contributing to product stability and hygiene without relying on more aggressive synthetic preservatives. Hinokitiol has also been the subject of extensive biological research. Studies have explored its interactions with cellular systems, focusing on mechanisms underlying its antimicrobial and bioactive effects. One notable aspect of its chemistry is its ability to chelate metal ions, a property that has been linked to some of its biological activities. This metal-binding capability has made hinokitiol a useful probe in studies examining the role of metal ions in biological processes and microbial growth. In addition to biological applications, hinokitiol has played a role in fundamental chemical research. Its unique structure has served as a model for studying non-benzenoid aromaticity, tautomerism, and hydrogen bonding in conjugated systems. Synthetic chemists have used hinokitiol and related tropolones as starting points for the development of new compounds, exploring how modification of the ring system affects chemical and biological behavior. The continued interest in hinokitiol reflects the convergence of natural product chemistry, applied science, and fundamental research. From its discovery in tree heartwood to its adoption in antimicrobial applications and its role as a model compound in organic chemistry, hinokitiol exemplifies how a naturally derived molecule can influence multiple scientific disciplines. Its enduring relevance highlights the value of studying and applying compounds that originate from natural sources but offer insights and utility far beyond their original context. References 2025. Lipid membrane composition modulates Hinokitiol's effects on keratinocytes and fibroblasts. Chemistry and Physics of Lipids. DOI: 10.1016/j.chemphyslip.2025.105511 2025. Dynamic ubiquitination networks in liver cancer: decoding E3 ligases and deubiquitinases as gatekeepers of therapeutic resistance. Medical Oncology, 42(8). DOI: 10.1007/s12032-025-02912-0 |
| Market Analysis Reports |
| List of Reports Available for Hinokitiol |