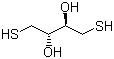
Dithioerythritol (DTE) is a small, four-carbon dithiol compound with the molecular formula C4H10O2S2. Structurally, it is a stereoisomer of dithiothreitol (DTT) and contains two thiol (-SH) groups on adjacent carbon atoms, which are key to its reducing properties. The compound is typically a white crystalline solid that is highly soluble in water and polar organic solvents such as ethanol or dimethyl sulfoxide. Its two thiol groups confer strong nucleophilicity and redox activity, which are essential for its chemical and biochemical applications.
The discovery of dithioerythritol stems from research into polyhydroxy compounds containing sulfhydryl groups. It was developed as a reducing agent to complement other dithiols, such as DTT, and to provide stereochemical variation in biochemical studies. The compound is particularly useful in studies of disulfide bond formation and cleavage in proteins, as it can selectively reduce disulfide bridges under mild conditions while maintaining protein stability.
Synthesis of dithioerythritol is generally achieved via the thiolation of erythritol derivatives. One common method involves the conversion of protected erythritol diols to the corresponding bis-thiol via substitution reactions with sulfur-containing reagents such as thionyl chloride followed by reduction with thiolates. The product is purified by recrystallization from polar solvents to obtain the stable, stereochemically defined DTE. The stereochemistry of DTE is important because it affects the reactivity and conformation of disulfide-containing biomolecules in which it is used.
Chemically, DTE functions as a strong reducing agent. The two vicinal thiol groups can reduce disulfide bonds to free thiols, forming an intramolecular disulfide in the process. This reversible oxidation-reduction reaction makes DTE an effective agent for maintaining sulfhydryl groups in their reduced form. Its reducing capacity is slightly lower than that of DTT, but it provides a useful alternative in cases where stereochemistry or reaction kinetics are critical. DTE also participates in nucleophilic reactions and can serve as a ligand for transition metals through its thiol groups.
In practical applications, DTE is widely used in biochemical and molecular biology research. It is employed to reduce disulfide bonds in proteins and peptides, helping to maintain or restore the native conformation of biomolecules. It is also used in enzyme assays, protein folding studies, and the preparation of samples for electrophoresis or mass spectrometry. Additionally, DTE can be used as a reagent in organic synthesis to selectively reduce disulfides or to introduce thiol functionality into small molecules.
Physically, DTE is stable when stored as a dry solid at low temperatures and should be protected from moisture, air, and light to prevent oxidation of the thiol groups. Handling precautions include the use of gloves and eye protection, as thiol compounds can have strong odors and may cause irritation. Solutions of DTE are usually prepared freshly before use to ensure maximum reducing activity.
Overall, dithioerythritol is a valuable vicinal dithiol with strong reducing properties and stereochemically defined structure. Its ability to selectively reduce disulfide bonds and maintain thiols in a reduced state makes it indispensable in biochemical research, protein chemistry, and certain synthetic applications. The compound’s combination of water solubility, redox activity, and stereochemical specificity allows it to function effectively in studies of protein folding, enzymology, and thiol-dependent reactions.
References
2025. Toxicological Effects of Air Pollutants on Human Airway Cell Models Using Air�liquid Interface Systems: A Systematic Review. Current Environmental Health Reports.
DOI: 10.1007/s40572-025-00491-w
|



















 GHS07 Warning Details
GHS07 Warning Details