Online Database of Chemicals from Around the World
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Ring Specialty Chemicals Inc. | Canada | Inquire | ||
|---|---|---|---|---|
 |
+1 (416) 493-6870 | |||
 |
info@ringchemicals.com | |||
| Chemical distributor | ||||
| chemBlink standard supplier since 2010 | ||||
| Winchem Industrial Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (574) 8385-1061 | |||
 |
info@win-chemical.com hhp13@hotmail.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Jadechem Ningbo Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (574) 8907-5960 +86 13777214302 | |||
 |
info@jadechem-colours.com | |||
| Chemical manufacturer since 2008 | ||||
| chemBlink standard supplier since 2012 | ||||
| Cangzhou Xincheng Weiye Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (317) 777-4995 | |||
 |
hailee@xcwychem.com | |||
| Chemical manufacturer since 1995 | ||||
| chemBlink standard supplier since 2019 | ||||
| Hubei Marvel-bio Medicine Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (027) 8773-8507 | |||
 |
maoerwo123@163.com | |||
 |
QQ chat | |||
 |
WeChat: kangbao813 | |||
| Chemical manufacturer since 2016 | ||||
| chemBlink standard supplier since 2025 | ||||
| Whyte Chemicals | UK | Inquire | ||
|---|---|---|---|---|
 |
+44 (20) 8346-5946 | |||
 |
sales@whytechemicals.co.uk | |||
| Chemical distributor | ||||
| Classification | API >> Synthetic anti-infective drugs >> Disinfectant antiseptic |
|---|---|
| Name | Basic Violet 1 |
| Synonyms | Methyl Violet; C.I. 42535; Methyl Violet 2B |
| Molecular Structure | 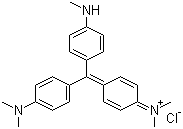 |
| Molecular Formula | C24H28ClN3 |
| Molecular Weight | 393.95 |
| CAS Registry Number | 8004-87-3 |
| EC Number | 616-846-4 |
| SMILES | CN=C1C=CC(=C(c2ccc(N(C)C)cc2)c2ccc(N(C)C)cc2)C=C1.Cl |
| Melting point | 137 ºC (Decomposes) (Expl.) |
|---|---|
| Solubility | Water: 45mg/mL, DNSO: 10 mM (Expl.), Water: soluble (Expl.) |
| Hazard Symbols |




 GHS05;GHS06;GHS07;GHS08;GHS09 Danger Details
GHS05;GHS06;GHS07;GHS08;GHS09 Danger Details | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H301-H302-H318-H319-H351-H400-H410 Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P203-P264-P264+P265-P270-P273-P280-P301+P316-P301+P317-P305+P351+P338-P305+P354+P338-P317-P318-P321-P330-P337+P317-P391-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||||||
|
Basic Violet 1, also known as crystal violet or methyl violet 10B, is a triphenylmethane dye with the molecular formula C25H30ClN3. Structurally, it consists of a central carbon atom bonded to three aromatic rings, two of which are substituted with dimethylamino groups, and one with a methyl group, forming a highly conjugated chromophore. The compound appears as deep purple to dark violet crystals that are highly soluble in water and polar organic solvents such as ethanol and methanol. Its intense color arises from the extensive π-conjugation and electron-donating amino groups, which allow absorption of visible light in the 580–600 nm range. The discovery of Basic Violet 1 dates to the late 19th century as part of the development of synthetic triphenylmethane dyes. It was initially synthesized as a derivative of aniline and formaldehyde reactions under acidic conditions, providing a vivid, stable dye for textiles. Over time, it became one of the most widely used basic dyes due to its bright coloration, ease of application, and stability under light and heat. Its use expanded beyond textiles into biological staining, ink production, and analytical chemistry. Synthesis of Basic Violet 1 involves the condensation of N,N-dimethylaniline with formaldehyde in the presence of hydrochloric acid to form the leuco intermediate. This intermediate is subsequently oxidized to yield the cationic dye. The chloride counterion balances the positive charge on the central carbon, resulting in the water-soluble salt form. The product is purified by crystallization or filtration to remove unreacted intermediates and by-products. Variation in the number of methyl groups on the amino substituents produces related methyl violet dyes with slightly different spectral properties. Chemically, Basic Violet 1 is a cationic dye with strong affinity for negatively charged substrates, such as anionic fibers, nucleic acids, and cell membranes. Its planar, conjugated structure allows intercalation and stacking interactions with DNA, making it useful in histology and microbiology. The dimethylamino groups enhance electron density, contributing to its intense coloration and basic character. It is generally stable under normal laboratory conditions but can degrade under strong oxidizing agents or prolonged exposure to light. In practical applications, Basic Violet 1 is primarily used as a textile dye for wool, silk, and nylon fibers. In biology, it is employed as a Gram stain component for bacterial classification, a nuclear stain in microscopy, and a marker for protein gels. It is also used in inks, paints, and as a pH indicator in certain analytical assays due to its color change properties under acidic and basic conditions. Additionally, Basic Violet 1 has applications in forensic science for fingerprint visualization and as a dye tracer in various laboratory experiments. Physically, Basic Violet 1 is stable when stored in a cool, dry environment, protected from light and moisture. Handling precautions include gloves, protective clothing, and eye protection, as concentrated dye solutions can stain skin and may cause irritation. Proper ventilation is recommended when working with powder forms to avoid inhalation. Overall, Basic Violet 1 is a versatile triphenylmethane dye with extensive applications in textiles, biological research, and analytical chemistry. Its intense color, water solubility, and cationic properties make it a valuable compound for staining, coloring, and experimental purposes in scientific and industrial contexts. References 2020. Cytotoxic Profiling of Annotated and Diverse Chemical Libraries Using Quantitative High-Throughput Screening. SLAS Discovery. DOI: 10.1177/2472555219873068 2019. A High-Throughput Screen of a Library of Therapeutics Identifies Cytotoxic Substrates of P-glycoprotein. Molecular Pharmacology. DOI: 10.1124/mol.119.115964 |
| Market Analysis Reports |
| List of Reports Available for Basic Violet 1 |